The electroretinogram (ERG) is a diagnostic test that measures the electrical activity of the retina in response to a light stimulus. The ERG arises from currents generated directly by retinal neurons in combination with contributions from retinal glia. List of Elements in Order of Electronegativity χ. List of elements ordered by electronegativity is listed in the table below with atomic number, chemical symbol and electronegativity value.
Electronegativity
When two atoms combine to form a bond, the position of bonding electron pair (hence nature of the bond) depends on the nature of the atoms. If the atoms are like, the electron pair is evenly shared. But if the bond is formed between two unlike atoms, usually, the electron pair is nearer to one atom than the other. This type of behavior of the atoms in molecules and some of their properties can only be interpreted if the atoms are assumed to have a tendency to attract electrons to themselves and also that some atoms have a greater tendency than others.
This assumption is further supported by the fact that in the bonds C-H, N-H, O-H, and F-H the percentage of ionic character increases and the bond pair gets closer to the first atom as we proceed from left to right. This tendency of atoms in molecules to attract electrons to themselves is called electronegativity. Thus, electronegativity is that atomic property which determines whether the bonding electrons are evenly or unevenly shared when that atom is in contact with many other atoms.
The first-hand information about the electron attracting tendencies of the atoms can be obtained from what is called an electronegativity scale. In an electronegativity scale, the electronegativity is a relative term. Such a scale can only be devised by taking some reference value. Arbitrarily, Pauling assigned a value of 4 for fluorine which is the most electronegative element. It must be born in mind that a particular numerical value given to the element does not represent the actual content of negative charge on it but it is the electron attracting tendency relative to the reference value.
Factors affecting electronegativity
Electronegativity of an element depends on many factors, some of which are as follows:
- Effective nuclear charge: An increase in effective nuclear charge increases the attractive force for a bonding electron pair. Thus increase or decrease in effective nuclear charge affects electronegativity in like manner.
- Oxidation state: As an increase in the oxidation state of an atom increases its ability to attract electron i.e. electronegativity also increases. Thus, we can say that an element will have greater electronegativity from its higher oxidation state and lower electronegativity from a lower oxidation state. For example, the acidic character of the oxyacids of chlorine increases with an increase in the oxidation state of chlorine. Increased tendency of proton release is a consequence of increased electronegativity of chlorine because now it withdraws the O: Cl bond pair to a greater extent making the O atom more electron deficient.
- Atomic size: The valance electrons of larger atoms will be attracted by weaker force. In other words, electronegativity increases when atomic size decreases and vice-versa.
- Hybridization state of atom: Electronegativity increases when s character in the hybrid orbitals increases because s electrons can penetrate more towards the nucleus and the bond pair will be attracted more by the nucleus in case of overlapping of orbitals have greater s character.
Periodic trends in electronegativity:
Variation in effective nuclear charge and atomic radii affect electronegativity in the same manner as they affect ionization energy. An increase in the effective nuclear charge and a decrease in the atomic radii will increase the attractive force which is equated to the electronegativity.- Variation in a period: Within a period, electronegativity increases with increasing atomic number. This is consistent with the excepted increase in effective nuclear charge and decreasing atomic radius from left to right. Thus, in a period the alkali metals are the least electronegative element (hence most electropositive and metallic) and halogens are most electronegative and hence most non-metallic in nature.
- Variation in a group: In a group, electronegativity decreases from top to bottom. Again this agrees with the trends excepted towards increasing atomic radii. Thus, in a group, the lighter elements coming at the top are most electronegative and those at the bottom are least electronegative.
Applications of electronegativity:
The uses of electronegativity can roughly be grouped intothe following categories:

Nature of bond: When two atoms of equal electronegativity combine to form a bond, the bond pair will be equally shared between them because the electron attracting tendency is equally pronounced in both the atoms. Thus H-H, Cl- Cl, O=O may be considered as pure covalent bonds. But if one atom has a very strong ‘desire’ to attract the electron than the other, it may snatch an electron from the latter to form an ionic bond. For example, the compound of halides with alkali and alkaline earth metals are ionic in nature.
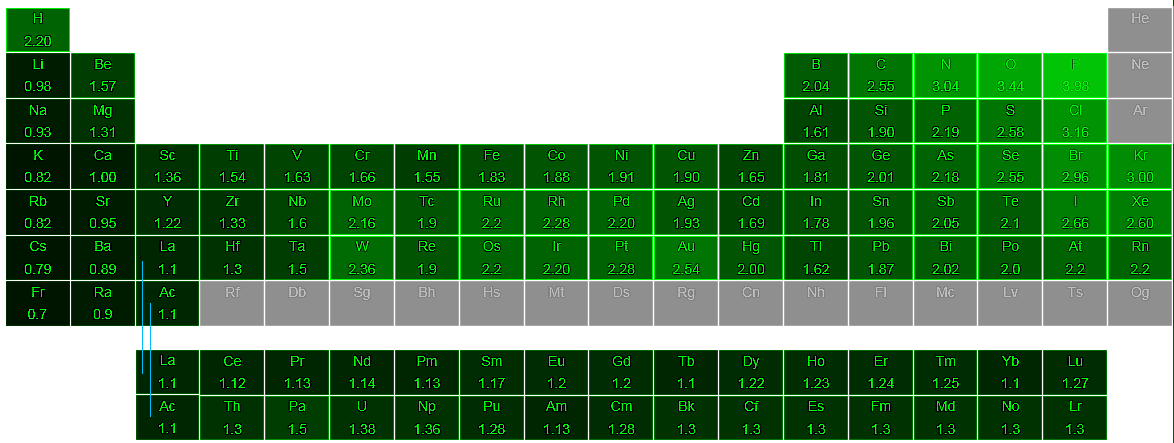
Electronegative Values
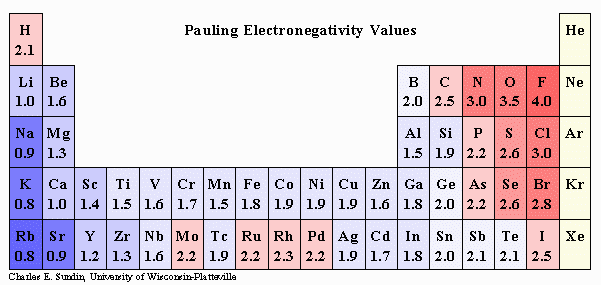
Electronegative Atoms
Polarity in covalent bond and explanation of dipole moment: Mostly the bonds are formed between atoms of unequal electronegativities but the difference is not sufficient enough to cause the ionic bond between them. Here no atom is capable of taking complete control of both the bonding electrons, though the bond pair is shifted towards the more electronegative atom. Consequently, the molecule becomes a dipole in which the positive and negative charges do not coincide.
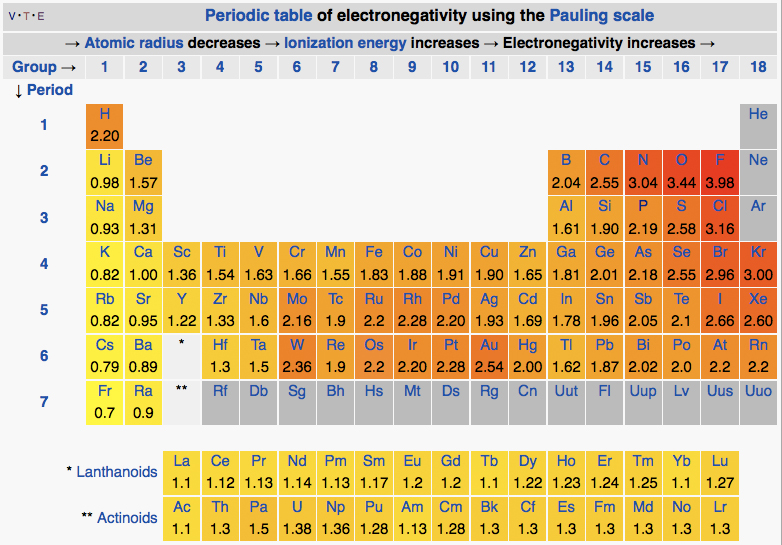
Electronegativity In Periodic Table
Calculation of bond length: When a covalent bond is formed between the two atoms of different electronegativity, the bond length between them is found to be slightly shorter than the sum of their covalent radii. An empirical equation for the calculation of bond lengths.
Actual bond distance = rA+ rB – 0.09(ꭕA -ꭕB)
where rA and rB are atomic radii and ꭕA and ꭕB are electronegativities of A and B.
Reaction Mechanism: The explanation of nucleophilic and electrophilic reactions is based on the assumption that separate centers of positive and negative charges exist within the molecules.
Ionic character in the covalent bond: Pauling proposed that there is a direct relationship between the difference of electronegativities of two atoms and ionic character in the covalent bond between them.
Nature of oxides: The nature ofoxides of an element may be determined on the basis of electronegativity ꭕA of the element and that of oxygen ꭕO. If this difference (ꭕO -ꭕA)is 2.1, the oxide will be amphoteric and if it is <2.1 then the oxide willbe acidic but if the difference is >2.1 then this oxide will be basic.
